Skeletal Muscle Tissue Engineering
Authors: Erik Burns, Kelsi Krier, Marc Lebel, Matt Peppel
Motivation
Damage to skeletal muscle tissue from injury, myopathies and other muscle diseases can lead to various complications for the human body. When muscle tissue is damaged, it loses its ability to function properly. In many cases mobility can become severely limited, causing the respiratory system to become restricted. Currently there are very few options for treatment. Tissue transfer allows for donor or autologous muscle tissues to be transplanted onto the damaged muscle tissue. However, current procedures for this treatment have often led to rejection by the body or incomplete regeneration. Few alternative treatments have led to consistent successful regeneration of muscle tissue. However, recent developments in skeletal muscle tissue engineering have offered promising results.
Skeletal muscle tissue engineering involves using the regenerative properties of satellite cells to “re-grow” damaged muscle tissue within the body. There are many engineering obstacles to overcome when trying to regenerate muscle tissue. Some of these obstacles include proper alignment of myofibrils with myosin and actin filaments, intracellular calcium-storage and acetyl-cholin receptors, biocompatibility, vascularization, and innervation of the new tissue. Although there have been major advances in skeletal muscle tissue engineering, there are still areas that need additional research and development. The long-term goal is to develop an autologous striated muscle implant capable of mirroring intact muscle's architecture and physiological functions.
Overview of Skeletal Muscle
The human muscular system is comprised of three different types of muscle tissue: cardiac, smooth and skeletal muscle. Together these three types of muscle make up about half of the body’s mass, and skeletal muscle alone makes up about 80% of the muscular system. Skeletal muscle falls under the categories of striated and voluntary muscle. A striated muscle is composed of alternating light and dark bands and voluntary muscle is controlled voluntarily by the somatic nervous system, however, there are parts of the skeletal muscle controlled involuntarily.
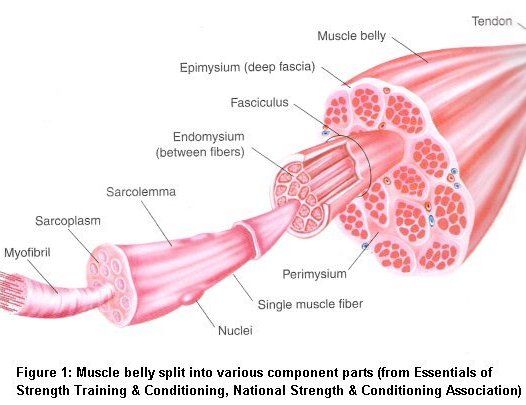
Structure
Skeletal muscle consists of various components working together to provide efficient function. Each muscle is made up of muscle tissue, connective tissue, nerve tissue and vascular tissue. Skeletal muscle is attached to the bone by tendons at each end. Skeletal muscle can range in size from very small and fine to long and wide.
Muscle Fiber
Every muscle is actually a bundle of many thousands of muscle cells, or muscle fibers, lying parallel to each other and surrounded by connective tissue. Interestingly, muscle fibers have multiple nuclei and plentiful mitochondria. The multiple nuclei arise due the specific ways in which an embryo develops. Muscle fibers need many mitochondria since they consume large quantities of energy. The size of muscle fibers is somewhat larger than normal cells. They usually have a long cylindrical shape and can reach lengths of multiple feet! Muscle fibers run the length of a muscle and are composed of a collection of smaller units called myofibrils.
Myofibril
Myofibrils make up the majority of a muscle fibers volume, up to 80%. They are also cylindrical in shape and run the entire length of the muscle fiber. Myofibrils have a striated appearance due to the arrangement of dark and light bands within each myofibril. These dark and light bands are known as the A and I bands respectively. The bands run parallel to each other and are created by alternating filaments known as thick and thin filaments. These thick and thin filaments are arranged hexagonally in relation to each other, thin filaments around thick filaments. Between each filament is a special cross bridge that plays an important role in muscle contraction.
A bands consist of both thick and thin filaments but only thick filaments run the entire length of the A band. Separate thin filaments protrude into the ends of the A band but do not run its entire length. The middle part of the A band that the thin filaments do not reach is known as the H zone. In the center of the H zone is a collection of proteins that act to support the thick filaments. This collection of proteins is called the M line.
I bands include thin filaments, but no thick filaments. The ends of thin filaments that are not within an A band act as the boundary of an I band. In the center of an I band is another line, the Z line, which is actually a cytoskeletal disc connecting parallel thin filaments. The region between two Z lines in a myofibril is the functional unit of a muscle known as the sarcomere. As the functional unit, the sarcomere is the smallest constituent of muscle that can still perform all of the functions of a muscle. The sarcomere includes one entire A band and two half I bands. Sarcomeres also increase muscle size. During exercise, sarcomeres are added to the ends of the myofibrils, thus increases the size of the muscle.
Another important component of the myofibril is titin. Titin is a very large protein that can be stretched while maintaining integrity. Within the myofibril, titin acts to stabilize the thick filaments correctly with the thin filaments. It is also important in muscle activity acting like a spring and allowing the muscle to be more elastic.

Myosin & Actin
Myosin is a special protein found only on thick filaments. Each thick filament may have hundreds of myosin molecules, all organized in a specific way. The easiest way to picture a myosin molecule is by comparison to golf clubs. The handles are actually intricate protein tails that twist around each other. At the ends are the club heads, which are really specialized binding sites for the protein actin and myosin ATPase. The heads are what make up the important cross bridges between thick and thin filaments. Each thick filament is just two of these myosin molecules lined opposite each other, connected at the “handles.”
Actin, along with tropomyosin and troponin make up the major parts of thin filaments. All three are proteins with actin being the most significant. Actin molecules are round in shape and form the spine of the thin filament. On each actin molecule is a special binding site where the heads on myosin molecules can attach. However, when a muscle fiber is relaxed, tropomyosin and troponin act to cover the binding sites on actin. Thus myosin cannot attach when the muscle fiber is relaxed. Tropomyosin is a long thin protein that covers the binding sites on actin. Troponin is able to bind to both tropomyosin and troponin as well as to Ca2+ and acts to move tropomyosin onto or off of the binding site on actin.
Contraction
Every type of muscle cell can contract. Smooth muscle controls the contraction of blood vessels, the gastrointestinal tract, and other organs throughout the body. Cardiac muscle is responsible for the pumping action of the heart. Skeletal muscle controls almost all of our movements. Muscle contraction can be thought of as the creation of tension from muscle fibers through communication with motor neurons. The cross bridge between actin and myosin plays a very important role in muscle contraction as does the action potential created by an action potential across the muscle cell membrane. Acetylcholine and Ca2+ also play significant parts in muscle contraction.
Sliding Filament Mechanism
Skeletal muscle contraction is achieved using the sliding filament mechanism. The process starts in the central nervous system where motor neurons transmit an action potential down their axons. This potential stimulates the release of acetylcholine across the synaptic cleft to the muscle cell membrane. Here the acetylcholine binds to special acetylcholine-gated channels. This binding generates an action potential which then travels down special tubules that form at the connection between each A and I band. These tubules, called T tubules, dip down into the muscle fiber. The potential activates the release of Ca2+ from within the sarcoplasmic reticulum. Ca2+ travels through the cytosol until it comes in contact with troponin. When this happens the Ca2+ binds to the troponin causing a conformational change. This change in shape pulls tropomyosin off of the myosin binding site on actin. With the binding site now free, myosin cross bridges bind to actin and energy from ATP allows it to pull the thin filament towards the center of the sarcomere.

Skeletal Muscle Degradation
Injuries
The most common injury seen in skeletal muscle tissue is a tear. Tears often occur when muscles are overworked or overstrained through activity. There are three different degrees of muscle tissue tears, two of which usually heal on their own. A first-degree muscle tear is a small tissue tear, such as a pulled muscle, while a second-degree tear is an almost complete tear across the muscle. Figure 1 depicts a first-degree tear. Most of the time, neither of these injuries requires surgery because the skeletal muscle that was damaged has the ability to regenerate itself. However, the process of regeneration is slow and the new muscle may never be as strong as it once was.
Figure 1.

A strain is a first-degree muscle tear. With rest, this muscle tissue will regenerate and strength will be normal again.
Third-degree muscle tears are complete tears across the tissue. Because these injuries damage a larger area of muscle, regeneration cannot usually occur. Surgery is almost always needed to stop internal bleeding and to re-attach the muscle before scar tissue begins to form, which would affect muscle function and regeneration ability in the future. Other wounds that would damage and possibly even deform the muscle, such as gunshot wounds, car accidents, or animal attacks, can also permanently injure or kill muscle. The damage that the muscles would sustain through these injuries could leave patients with missing muscle tissue that would not restore itself. If skeletal muscle was damaged beyond repair and could not heal itself, implantation of artificial skeletal muscle would be an option to regain muscle tissue, function, and strength.
Myopathies
Myopathies are neuromuscular diseases that progressively cause muscle fibers to no longer function and in turn cause muscle weakness. All of these diseases damage muscle tissue continuously, until the muscles are so weak they die and mobility is lost. Most of these muscle disorders occur before a patient is in their 20’s, but some do not appear until later in life.
Muscular dystrophies are a group of hereditary diseases that cause gradual muscle weakness because muscles are no longer able to regenerate. In most cases, muscle tissue degenerates quickly and muscles gets so weak that the disease becomes debilitating and a patient requires a wheelchair to move. As with almost all myopathies, when muscles degrade, connective tissue forms in place.[11] Figure 2 shows a slide of muscle tissue from a patient who died from a muscular dystrophy called Duchenne Muscular Dystrophy, or DMD. There is less muscle tissue, the red tissue, than there is of the white tissue, the connective tissue.[6] Of the nine different types of muscular dystrophies, disease-causing defective genes have been identified in about four. Muscular dystrophy affects an estimated 55,000 people nationwide.[13]
Figure 2.

"Histopathology of gastrocnemius muscle from patient who died of pseudohypertrophic muscular dystrophy, Duchenne type.
Cross section of muscle shows extensive replacement of muscle fibers by adipose cells." Dr. Edwin P. Ewing, Jr. [5]
There are many different types of myopathies that have been discovered and studied as of today. A few of the most common are congenital myopathies, mitochondrial myopathies, inflammatory myopathies, and metabolic myopathies. Congenital myopathies are hard to detect because changes are shown at the microscopic level. As the disease progresses, muscles lose their contractile ability. Muscle weakness, slow reflexes, motor and speech skills are also symptoms of congenital myopathies.[10] Mitochondrial myopathies are caused when mitochondria, which are essentially a cell’s power plant, is damaged by genetic defects. Without mitochondria to produce energy, muscles cannot function properly and become weaker as time goes on.[14] Myopathies that cause continuous swelling to the muscle tissue are called inflammatory myopathies. These are thought to be autoimmune diseases in which the white blood cells no longer defend against infection, but instead attack the muscle tissue. Constant inflammation of tissues may damage the blood vessels that run through tissue, causing weakness and possible tender-to-the-touch muscles.[12] Metabolic myopathies are caused by defects in enzymes, in which these enzymes are not able to complete their pathways or cellular reactions.[7],[4] An example of this disease is when glycogen cannot be stored effectively or broken down in tissues.
The myopathies discussed above cause degeneration of muscles and increasing weakness throughout life. As of today, there is no treatment for any muscle degenerative diseases. Skeletal muscle tissue engineering could be utilized as an alternative to rebuild lost or damaged muscles.
Spinal Muscular Atrophy
Spinal muscular atrophy is a disease that occurs when the survival motor neuron gene, SMN1, is missing in a patient’s genetic makeup. Neurons in the central nervous system, called motor neurons, are responsible for controlling muscle movements.[17] When present, the SMN1 gene produces proteins necessary to the motor neurons. When the protein cannot be made, nerves in the spinal cord die and muscles are not able to function. Muscle tissue engineering, in association with gene therapy, could battle the disease effectively by restoring muscle and providing mobility.

DNA transcription and translation in a patient with spinal muscular atrophy. A deficiency of the SMN1, illustrated here by SMN2, will produce low protein levels and can not feed the motor neurons with nutirients.
Past Treatments
The complex procedures in skeletal muscle tissue engineering today could never exist without the methods of muscle reparation that were available at the turn of the 20th century. These methods, including physical therapy and muscle transplantation, are still available and are widely used by the public.
Physical Therapy
Physical therapy is a branch of rehabilitative health that uses specially designed exercises and equipment to aid patients in restoring or improving their physical ability. The practice of physical therapy became popular in the United States after World War I when there was a great need for rehabilitation of wounded soldiers. Physical therapists work with a vast array of patients including ones with musculoskeletal birth defects, muscle injuries, and stroke complications. Physical therapists aim to restore physical ability through techniques such as exercise, heat, cold, and electrical stimulation.
Though there are several techniques in promoting improvement of physical ability, the primary technique for promoting muscle growth is physical exercise. When an injured or degraded muscle is targeted, specific exercises using resistance will be employed. Doing exercise will cause small rips in the myofibrils of the targeted muscle to occur. In a process called muscular hypertrophy, the myofibers are polynucleated by satellite cells. The satellite cells then fuse to the already matured muscle cell and signal for the production of sarcomeric proteins such as actin and myosin. The proteins that are synthesized in this process repair the damaged tissue and make them larger and stronger. As a result of exercise, muscle tissue can grow larger through hypertrophy; however, muscle cell count is not improved. Muscular hypertrophy does not stimulate muscle cells to divide; it just promotes growth at the sarcomeric level.
Muscle Transplantation
Muscle Transplantation is the physical transposition of muscle cells to damaged muscles caused by injury or disease. Since 1970, as a result of improved microneurovascular techniques, the transplantation of large muscles has been made feasible. Muscles over six grams in mass can be transplanted directly to an injured site as a myofascial flap. In humans, fascia is the interconnective tissue web that permeates the body. The fascia penetrates and surrounds muscles, nerves, and capillaries to provide support and stability for such imperative structures to human function. This myofascial flap which is grafted from another muscle in the body may be transposed to the injured site with or without the vasculature intact. If the vasculature is not intact, microneuralvascular repair will be required in order for the muscle to become functional. Since muscles exhibit great flexibility it is not important to match fiber types of the donor and recipient tissue. When the myofascial flap is ready to be transposed, the tendons are severed and sutured to the new tendon stumps. Depending on the type of transplant, nerves may be directly transplanted or can be repaired post-surgery. Though this method for muscle transplantation has been proved to be effective for patients suffering from disease or injury, the new transplanted muscle can only exert up to 40% of its’ original force. For this reason, the new muscle can only be used for normal movement and mobility. Large loads of stress on the transplanted muscle can cause further damage and trauma.





Skeletal Muscle Tissue Engineering
Skeletal muscle tissue has a structure that is regulated by nutrition, hormones, electrical activity, and tension. During all stages of development, muscle cells are exposed to mechanical force in order to organize mononucleated myoblasts into parallel, multinucleated myofibers, and also match bone and muscle growth rate. The active study of these processes has led to the engineering of muscle organoids. These organoids, while synthetic, can be used as a replacement for skeletal muscle tissue if they are capable of performing functional work based on size and packing density of myofibers. These organoids must also display characteristics of in vivo muscle such as parallel orientation of myofibers organized into fascicles with tendon-like ends. Of course, the bioartificial skeletal muscle must have the capacity for calcium storage and must be vascularized and innervated, in order to complete the sliding filament mechanism used when contracting muscles. Human skeletal muscle stem cells (usually satellite cells are preferred to other myogenic cell lines such as C2C12 or L6) can be easily isolated from muscle and expanded into culture using muscle biopsy. From the myoblast cell cultures, the cells can be either implanted into a biocompatible 3D matrix or appropriate transport matrix in order to initiate differentiation. In in vitro engineering, muscle tissue is generated first then implanted into the patient. Conversely, in in vivo engineering, the patient is used as the ‘bioreactor’ and the muscle tissue is generated inside the patient.
Both methods of skeletal muscle tissue engineering require use of a cell matrix. There are many demands that the scaffolds must meet. Successful matrices should of course provide an appropriate surface for cell adhesion and growth, which includes proliferation and differentiation. Obviously, the scaffold must be nonimmunogenic and should be degradable, in order to maintain a high ratio of contractile/restrictive components within the muscle construct. Nonwoven meshes of polyglglocic acid (PGA) fibers are appealing cell delivery materials due to a large surface area/volume ratio and its ability to be easily molded into different structures. [17] Studies have been conducted using polymer scaffolds to determine the cellular effects of mechanical stimulation. Devices have been created a mechanical stimulator capable of stretching and relaxing in vitro cell cultures. The average myofiber diameter and elasticity increased as a result of the mechanical stimulation. Other studies have indicated that artificial skeletal muscle functioned differently from native tissue without mechanical stimulation. Electrical stimulation is also important in inducing the appropriate functions of native skeletal muscle. Just like nerve stimulation that occurs during myogenesis and regeneration of injured tissue, electrical stimulation is shown to induce differentiation of neural activity
There is some debate as to which material is most suitable for use as a transport matrix for clinical application of skeletal muscle engineering. Matrigel (R) is a commonly used matrix. This gel protein mixture is derived from mouse tumor cells and contains unregulated (and unknown) composition of proteins and growth factors which is a potential hazard that limits the utility for practical application. PLGA models can induce a strong foreign response, which doesn't meet the important characteristic of biocompatibility. One of the preferred constructs is derived from fibrin, which is reabsorbable, nonimmunogenic, and even clinically approved.
In Vitro Engineering

The in vitro fabrication of artificial mature muscle has many benefits over delivering isolated precursor cells. We can pre-engineer custom tissue conformation in order to complete precise repair at the specific injury site. Using a 3D matrix, we can also locally deliver growth factors upon implantation. Internally or externally generated muscle stimulation is an important element of aligning and fusing the myoblasts during formation of the organoid. These forces also have an important impact on myofiber diameter, composition and cell number in mature skeletal muscle tissue.
In vitro skeletal muscle tissue engineering is done by culturing satellite cells of primary myoblasts isolated from muscle in an environment complimentary to forming a three dimensional tissue construct. In this 3D matrix, the cells differentiate. The final step is implantation of the generated tissue construct into the patient.
One promising approach for designing a biomimetic tissue uses biocompatible hydrogels. Natural hydrogels are superior to synthetic ones thanks to a higher cell attachment site density which is beneficial to creation of a 3D matrix since the cells can spread throughout the construct. Hydrogels encourage uniform and dense cell entrapment which ultimately leads to higher cell density. Mechanical forces can also control the alignment of cells throughout the construct. With the aid of computer design such as photo-lithographic and soft-lithographic pattering, hydrogels have become much easier to mold into appropriate three-dimensional shape. [3]
One of the biggest obstacles facing skeletal tissue engineering is vascularization. A microsurgical atrioveneous-loop (AV-loop), which was first used to vascularize a full-thickness skin graft nearly twenty years ago, can be implemented into the biocompatible matrix in order to pre-vascularize the construct. It has been shown that using this technique preserves the myogenic properties and promotes survival of the myoblasts while vascularizing the neo-muscle inside the host. [2]
In order to restore the injured host muscle function upon transplantation, the engineered tissue must be able to generate adequate amount of active force. Creating a native-like tissue architecture equipped with growth factors can enhance the production of force. Different applications of strain have different effects during skeletal muscle growth in vitro. Myoblasts are aligned and fused into myotubes when subject to axial strain. Cyclic strain increases the proliferation rate of myoblasts which increases cell density. Collagen cross-linking and decrease of elasticity occur easily in static cultures. [17]
In a study performed by Riboldi et al., electrospinning was carried out on a degradable polyesterurethane block known as DegraPol(R) using mouse C2C12 progenitor cells in order to fabricate a biomaterial scaffold. The electrospun scaffold was apt at adhering, proliferating, and differentiating the C2C12 line cells. Multinucleated myosin heavy chain expressing myotubes were aligned in the correct direction with the scaffold fibers after 3 days of the study. By aligning these myofibers, the organoid was able to provide sufficient contraction force. While very promising, this study revealed that the high tensile modulus and low-yield elongation of this particular microfibrous scaffold would be unable to stand up to the mechanical stimulation necessary to culture muscle tissue. [8]
The inclusion of myotendinous junction (MTJ) interface during in vitro engineering allows for production of organoids with acceptable muscle-tendon interfaces that are integrated when implanted into the body. [9] Insulin-like growth factor 1 (IGF1) is known as an inhibitor of skeletal muscle loss. When engineering the bioartificial organoids, scientists can include IGF1 in order to significantly increase myofiber production and area. The IGF1 infused organoids can produce an anabolic paracrine affect on the affected muscle, thus stimulating muscle growth. [15]
In Vivo Engineering


The second approach considered by engineers is to use in vivo engineering of skeletal muscle. This method also requires a muscle biopsy followed by a culturing of myoblast cells. Instead of differentiating the cells in a 3D scaffold, myoblasts are meshed with a biocompatible transport matrix and implanted directly into the patient. In this case, the patient is used as the 'bioreactor,' and cell differentiation and generation of muscle tissue takes place in vivo.

One of the major issues with skeletal muscle engineering is detecting the implanted cells inside the patient. The fate of the cells are determined by using Y-chromosome detection. In a study by Brier et al., a fibrin matrix was used to culture myoblasts derived from male rats. The cells were to be implanted into a female rat with a muscle defect. When transplanted into the area with the affected muscle, immunostaining showed that the cells retained the myogenic capabilities and the Y-chromosome hybridization in situ. This indicated the transplanted male myoblasts were integrated and able to survive in the female recipient. The donor cells could be detected up to 50 days after first injection. Successfully, the scientists were able to incorporate myoblasts into host myoblasts which resulted in the regeneration of skeletal muscle tissue. [1]
Using a small number of cells which differentiate in vivo can avoid in vitro engineering difficulties such as lack of sufficient vascularization of the large implanted organoid. Further progress in stem-cell research and technology would be very beneficial for this method of skeletal muscle tissue engineering.

Beier J.P., Kneser U., Stern-Strater J., Stark G.B., Bach A.D., Y chromosome detection of three-dimensional tissue-engineered skeletal muscle constructs in a syngeneicrat animal model, Cell Transplant, 13: 45-53, 2004
Because muscle maturation is directed by neurons, neurotization of synthetic organoids increases the possible contractile force. Directed control, via the nerves, is also possible if neuromuscular junctions (NMJs) develop after neurotization. In vivo vascularization can be accomplished by including releasable angiogenic factors such as vascular endothelial growth factor (VEGF), or recombinant human growth hormones in order to produce the proteins necessary to create capillaries in muscle tissue. [5]
Limitations
Despite years of development, reliable methods to uniformly align muscle cells in a large 3D scaffold are difficult to achieve. One major hampering aspect of skeletal muscle tissue engineering is being able to vascularize the tissue. The high metabolic demand of oxygen and other nutrients is difficult to match using today’s constructs. In the years to come, scientists must further understand the appropriate conditions necessary for adult satellite cell proliferation and differentiation. Small laboratory experiments involving rats or birds have been very promising. However, many of these studies do not directly translate to complete clinical application. Production of fully artificial autologous skeletal muscle tissue capable of restoring muscle function after a muscle injury will not be attainable until these aspects are cleared up.
If muscles are damaged or degenerating and an artificial skeletal muscle tissue implant would be ideal, there are many aspects that are conisdered. As discussed, small scaffolds are difficult to construct; larger constructs are even harder. It is a goal of muscle tissue engineering to possibly return muscle function to patients who use prosthetics, which will require a large muscle tissue to be grown. In the event that scientists are able to grow larger-sized artificial muscles, there are still limitations on the cost of implant and surgery and a doctor willing to perform the surgery.
Citations
1. Bach, A.D., and J.P. Brier, J. Stern-Staeter, R.E. Horch. "Skeletal Muscle Tissue Engineering." Journal of Cellular and Molecular Medicine 8(2004): 413-422.
2. Bach, A.D. et al. "A New Approach to Tissue Engineering of Vascularized Skeletal Muscle." Journal of Cellular and Molecular Medicine 10(2006): 716-726.
3. Blan, Weining, and Nenad Bursac. "Tissue Engineering of Functional Skeletal Muscle: Challenges and Recent Advances." IEEE Engineering in Medicine and Biology Magazine October 2008: 109-113.
4. Baylor College of Medicine. "Metabolic Myopathies." Department of Neurology. 19 November 2008. Baylor College of Medicine. 10 December 2008 <http://www.bcm.edu/neurology/patient_education/ndc/myopathies.html>.
5. Dhawan, Vikas et al. "Neurotization Improves Contractile Forces of Tissue-Engineered Skeletal Muscle." Tissue Engineering 13(2007): 2813-2821.
6. Ewing, Edwin P. Duchenne Muscle Dystrophy. 1972. US Department of Health and Human Services. 10 Dec. 2008 <http://phil.cdc.gov/phil/home.asp>.
7. Katirji, Bashar. "Metabolic Myopathies." EMedicine. 10 January 2007. Medscape. 10 December 2008 <http://emedicine.medscape.com/article/1173338-overview>.
8. Kumar, Challa. Tissue, Cell and Organ Engineering. Weinheim: Wiley-VCH, 2007.
9. Larkin, Lisa M. et al. "Structure and Functional Evaluation of Tendon-Skeletal Muscle Constructs Engineered in Vitro." Tissue Engineering 12(2006): 3149-3158.
10. Lopate, Glenn. "Congenital Myopathies." eMedicine. 5 January 2007. Medscape. 9 December 2008 <http://emedicine.medscape.com/article/1175852-overview>.
11. Mayo Clinic. "Muscular Dystrophy." Muscular Dystrophy. 8 December 2007. Mayo Foundation for Medical Education and Research. 10 December 2008 <http://www.mayoclinic.com/health/muscular-dystrophy/ds00200>.
12. National Institutes of Health. "Inflammatory Myopathies Fact Sheet." National Institute of Neurological Disorders and Stroke. 28 October 2008. National Institute of Neurological Disorders and Stroke. 10 December 2008 <http://www.ninds.nih.gov/disorders/inflammatory_myopathies/detail_inflammatory_myopathies.htm>.
13. National Institutes of Health. "Muscular Dystrophy: Hope Through Research." National Institute of Neurological Disorders and Stroke. 15 September 2008. National Institute of Neurological Disorders and Stroke. 10 December 2008 <http://www.ninds.nih.gov/disorders/md/detail_md.htm>.
14. National Institutes of Health. "NINDS Mitochondrial Myopathies Information Page." National Institute of Neurological Disorders and Stroke. 4 May 2007. National Institute of Neurological Disorders and Stroke. 10 December 2008 <http://www.ninds.nih.gov/disorders/mitochondrial_myopathy/mitochondrial_myopathy.htm>.
15. Shansky, Janet et al. "Paracrine Release of Insulin-Like Growth Factor 1 from a Bioengineered Tissue Stimulates Skeletal Muscle Growth in Vitro." Tissue Engineering 12(2006): 1833-1841.
16. Sherwood, Lauralee. Human Physiology. Belmont, CA: Thomson Higher Education. 2007. 253-263.
17. Saxena, Amulya et al. "Skeletal Muscle Tissue Engineering Using Isolated Myoblasts on Synthetic Biodegradable Polymers: Preliminary Studies." Tissue Engineering 5(1999): 525-531.
18. U.S. National Library of Medicine. "Spinal Muscular Atrophy." Medline Plus. 03 August 2008. U.S. National Library of Medicine. 10 December 2008
<http://www.nlm.nih.gov/medlineplus/spinalmuscularatrophy.html>.
19. Yan, Wentao, et al. "Tissue Engineering of Skeletal Muscle." Tissue Engineering 13(Nov 2007): 2781-2790.
Comments (3)
Rodrigo Gonzalez said
at 7:44 pm on Dec 14, 2008
The Wiki is very informative and looks like you all did a thorough job researching everything. Good Job!
Anonymous said
at 1:39 am on Dec 16, 2008
very thorough and clear, the diagrams of the in vivo and in vitro made it easy to understand the different ways to apply tissue engineering. thanks!
Anonymous said
at 1:41 am on Dec 16, 2008
After reading the article on engineered meat, I was wondering to what extent is there collaboration between these two areas of research? It sounds like engineering skeletal tissue requires a bit more specificity, but the processes seem quite similar.
You don't have permission to comment on this page.